magnesium sulfate can be made by reacting magnesium metal with which acid?
Yes When Magnesium reacts with hydrochloric acid according to the equation. You can make magnesium sulfate7-water in the laboratory by reacting magnesium - oxide with dilute.

Boardworks Ltd 20071 Of 55 2 Of 55 C Boardworks Ltd 2007 Chemical Reactions Additional Science Chemistry Jokes
In either case the oxide is reacted with sulfuric acid toproduce magnesium sulphate.

. Hydrochloric acid produces magnesium chloride. Magnesium sulfate react with carbon as reducing agent to produce magnesium oxide sulfur dioxide and carbon dioxide. The products other than magnesium oxide are gaseous and escapes leaving behind pure magnesium oxide. Soluble salts are formed by reacting metal oxides with acids.
Synthetically prepared magnesium sulfate is sold as Epsom salt MgSO 4 7H 2 O. As such its safer to synthesise it by reacting the oxide or carbonate with the acid or simply buying it commercially it. The products that are formed will be magnesium salt water and carbon dioxide. Magnesium reacts with hydrochloric acid according to the equation.
2 Calcium nitrate contains the ions Ca2 and NO 3 Give the formula of calcium nitrate. Magnesium sulfate is obtained from the mineral epsomite a white solid. What is the name of the gas made when magnesium reacts with sulfuric acid. Similarly does magnesium sulfate react with acid.
This is for interest only. Answer 1 of 2. Magnesium metal dissolves readily in dilute sulphuric acid to form solutions containing the aquated MgII ion together with hydrogen gas H2. Mg s 2 HCl aq -- MgCl 2 aq H 2 g This demonstration can be used to illustrate the characteristic reaction of metals with acid a single replacement reaction or to demonstrate of the generated hydrogen gas.
Corresponding reactions with other acids such as hydrochloric acid also give the aquated MgII ion. You can make magnesium sulfate-7-water in the laboratory by reacting magnesium oxide with dilute sulfuric acid. Acid metal salt hydrogen. Unless your syllabus specifically mentions reactions between metals and nitric acid you dont need to know this.
Acids react with metals to produce a salt and hydrogen. Acids will react with reactive metals such as magnesium and zinc to make a salt and hydrogen. The hydrogen causes bubbling during the reaction and can be detected using a burning splint which. When calcium carbonate and acetic acid vinegar combine a chemical reaction takes place and carbon dioxide gas CO2 is released.
1 Give one other type of substance that can react with an acid to form a soluble salt. It has a role as an anticonvulsant a cardiovascular drug a calcium channel blocker an anaesthetic a tocolytic agent an anti-arrhythmia drug an analgesic and a fertilizer. A gas is also produced. 0 1 1 mark.
Reacting metallic magnesium with acetic acid dissolved in dry benzene causes magnesium acetate to form along with the release of hydrogen gas. O crystals are obtained by evaporation. Reaction of acids 1. It is a magnesium salt and a metal sulfate.
Hydrochloric acid magnesium magnesium. They may be recrystallised to increase purity. Mgs 2 HClaq -- MgCl 2 aq H 2 g This demonstration can be used to illustrate the characteristic reaction of metals with acid a single replacement reaction or to demonstrate the generation of hydrogen gasThe flammability of hydrogen gas can be demonstrated by carefully holding a match or fireplace lighter up to. Acid reactions with metals.
Will magnesium sulfate react with zinc. 1 mark 0 1. This reaction takes place at a temperature of 700-900C. Does calcium sulfate bubble in vinegar.
Exactly what salt is made depends the acid used. Magnesium sulfate is a magnesium salt having sulfate as the counterion. No Zn is less reactive than Mg. Reaction of magnesium with acids Magnesium metal dissolves readily in dilute sulphuric acid to form solutions containing the.
MgOs H 2SO 4aq MgSO 4aq Magnesium sulfate7-water MgSO-47H. 3 Describe a method to make pure dry crystals of magnesium sulfate. Magnesium oxalate will react with sulfuric acid to form magnesium sulfate and oxalic acid. Sulfuric acid is an irritant.
You can get hydrogen from very dilute nitric acid with a reactive metal like magnesium but even in that case you will get some nitrogen oxides formed as well. It can also be prepared commercially by the reaction of magnesium carbonate MgCO 3 with sulfuric acid H 2 SO 4. Magnesium sulfate can be made by reacting magnesium metal with an acid. By direct reaction dissolving the metal power or strips in dilute acid though the reaction is exothermic and produces potentially explosive hydrogen gas.

Chemistry Education에 있는 Mrs Calverley님의 핀
What Happens To A Strip Ribbon Of Magnesium Over Time As It Reacts With Hydrochloric Acid What Precipitate Would Form What Visible Changes Would The Experimenter See With The Magnesium Quora
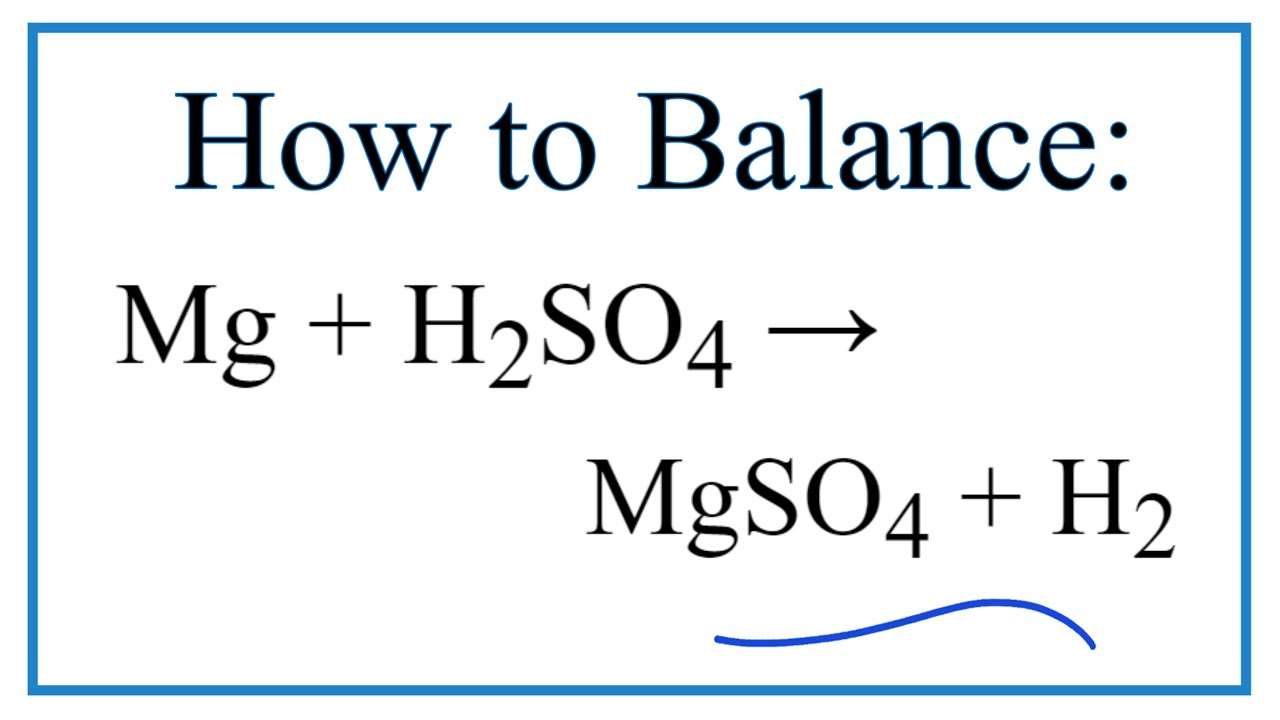
How To Balance Mg H2so4 Mgso4 H2 Magnesium Dilute Sulfuric Acid Youtube



Posting Komentar untuk "magnesium sulfate can be made by reacting magnesium metal with which acid?"